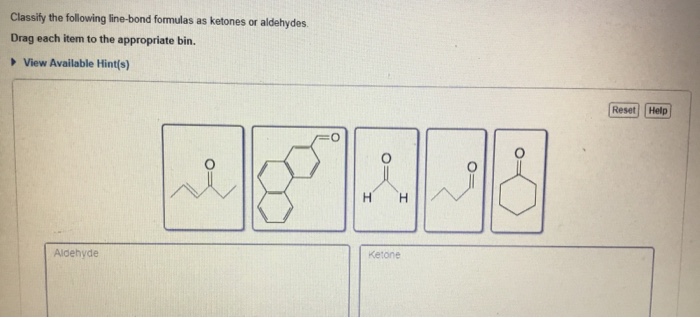
Classify the following line-bond formulas as ketones or aldehydes – In the realm of organic chemistry, ketones and aldehydes stand as two important functional groups with distinct characteristics and reactivity. This comprehensive guide delves into the intricacies of classifying line-bond formulas as ketones or aldehydes, empowering students and researchers alike with a clear understanding of these fundamental concepts.
Through a systematic approach, we will explore the structural features, general formulas, and key differences between ketones and aldehydes. Guided by a structured method, you will master the art of classifying line-bond formulas, enabling you to navigate the complexities of organic chemistry with confidence.
Ketones and Aldehydes: Classification and Structural Features
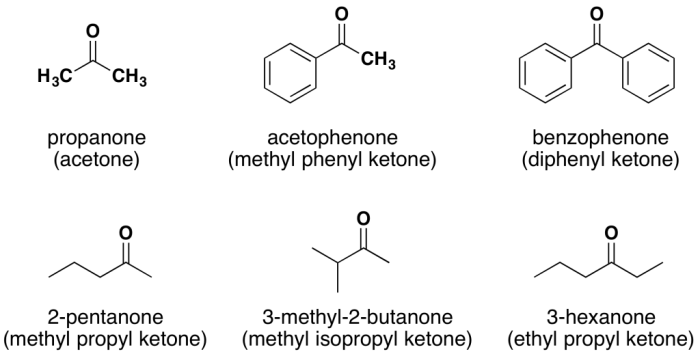
Ketones and aldehydes are two important functional groups in organic chemistry. They share a common structural feature, the carbonyl group (C=O), but differ in their connectivity to other atoms or groups. Understanding the structural differences between ketones and aldehydes is crucial for classifying organic compounds and predicting their reactivity.
Ketones, Classify the following line-bond formulas as ketones or aldehydes
Ketones are characterized by a carbonyl group bonded to two carbon atoms. The general formula for ketones is R-CO-R’, where R and R’ represent alkyl, aryl, or other organic groups. The carbonyl carbon in ketones is sp 2hybridized and forms a trigonal planar geometry.Examples
of ketones include:
- Acetone (CH 3-CO-CH 3)
- 2-Butanone (CH 3-CO-CH 2-CH 3)
- Cyclohexanone (C 6H 10O)
Aldehydes
Aldehydes are characterized by a carbonyl group bonded to one carbon atom and one hydrogen atom. The general formula for aldehydes is R-CHO, where R represents an alkyl, aryl, or other organic group. The carbonyl carbon in aldehydes is also sp 2hybridized and forms a trigonal planar geometry.Examples
of aldehydes include:
- Formaldehyde (H-CHO)
- Acetaldehyde (CH 3-CHO)
- Benzaldehyde (C 6H 5-CHO)
Classification Criteria
The key structural difference between ketones and aldehydes lies in the number of carbon atoms bonded to the carbonyl group. Ketones have two carbon atoms bonded to the carbonyl group, while aldehydes have only one carbon atom and one hydrogen atom bonded to the carbonyl group.To
classify a given line-bond formula as a ketone or aldehyde, identify the atom(s) bonded to the carbonyl carbon. If the carbonyl carbon is bonded to two carbon atoms, it is a ketone. If the carbonyl carbon is bonded to one carbon atom and one hydrogen atom, it is an aldehyde.
Practice Exercises
| Line-Bond Formula | Classification | Explanation |
|---|---|---|
| CH3-CO-CH3 | Ketone | The carbonyl carbon is bonded to two carbon atoms. |
| CH3-CHO | Aldehyde | The carbonyl carbon is bonded to one carbon atom and one hydrogen atom. |
| C6H5-CO-CH3 | Ketone | The carbonyl carbon is bonded to two carbon atoms. |
| H-CHO | Aldehyde | The carbonyl carbon is bonded to one carbon atom and one hydrogen atom. |
By understanding the structural differences between ketones and aldehydes, we can effectively classify organic compounds based on their line-bond formulas. This classification is essential for predicting their chemical properties and reactivity in various reactions.
Popular Questions: Classify The Following Line-bond Formulas As Ketones Or Aldehydes
What is the general formula for ketones?
The general formula for ketones is R-CO-R’, where R and R’ represent alkyl or aryl groups.
How can I distinguish between ketones and aldehydes based on their line-bond formulas?
In a line-bond formula, ketones have a carbon atom double-bonded to an oxygen atom and single-bonded to two other carbon atoms, while aldehydes have a carbon atom double-bonded to an oxygen atom and single-bonded to one hydrogen atom and one other carbon atom.
What are some examples of ketones?
Examples of ketones include acetone, butanone, and cyclohexanone.